ivWatch
Patient IV Monitoring
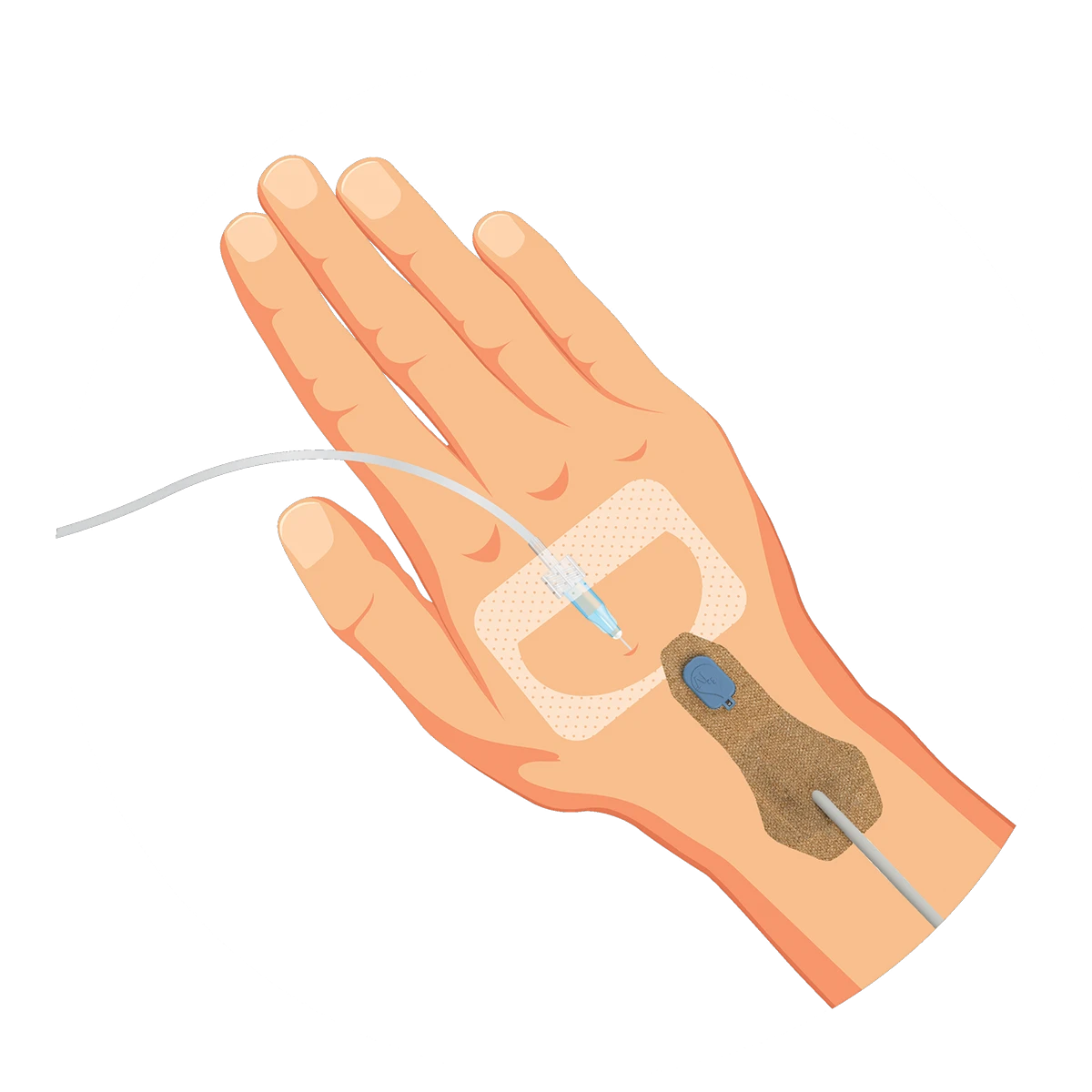
ivWatch was founded with one goal – to help clinicians provide the highest quality of care to every patient by detecting peripheral IV (PIV) infiltrations and extravasations at their earliest stage, when patient harm is highly preventable. Our non-invasive technology continuously monitors your patient’s IV status using advanced optics to notify you in real-time to the tissue changes that indicate a potential infiltration. ivWatch is here to help clinicians deliver optimal outcomes in vascular therapy.


Helm, R. E., Klausner, J.D., Klemperer, J.D., Flint, L.M., & Huang, E. (2015). Accepted but Unacceptable: Peripheral IV Catheter Failure. Journal of Infusion Nursing, 38(3), 189-203.
ivWatch offers Real-Time IV Monitoring so your team can:

Reduce Negative Patient Events
Every infiltration is a medicine dosing error that can lead to complications. ivWatch’s advanced signal processing can detect infiltrations in as little as 0.2 mL of IV fluid, with an average detection volume between 2 and 3 mL[1,2]—often well before it’s visible to the human eye.
1. uWatch, LLC. (2014, June). ivWatch Model 400: Device validation for infiltrated tissues. ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT02123745. ClinicalTrials.gov Identifier: NCT02123745
2. ivWatch, LLC. (2019, April). ivWatch SmartTouchTM Sensor: Device validation for infiltrated tissues. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04064229. ClinicalTrials.gov Identifier: NCT04064229

Improve Response Times
The ivWatch Monitoring system can detect infiltrations up to 15 hours before the clinician.[3] The system’s visible and audible notifications provide immediate notification, allowing the clinician to make a decision and respond to infiltrations early, in light of the multitude of other tasks they must manage.
3. Doellman, D. and Rineair, S. (2019). The use of optical detection for continuous monitoring of pediatric IV sites. Journal of the Association for Vascular Access: Summer 2019, 24 (2).

Enhance Workflow Efficiency
Clinicians can digitally document an IV status during routine patient assessments—ensuring seamless transition and patient information sharing between shifts. The historical IV log can also be captured into an EMR system.
4. ivWatch, LLC. (2014, June). ivWatch Model 400: Device validation for non-infiltrated tissues. ClinicalTrials.gov. https://clinicaltrials.gov/show/NCT02120443. ClinicalTrials.gov Identifier: NCT02120443

Decrease Liability
Every infiltration is a potential liability to both clinicians and the facility. The ivWatch Patient Monitoring System may help manage and mitigate liability concerns while reducing the incidence of patient harm.

Overcome Communication Barriers
ivWatch is constantly monitoring peripheral IV status throughout every stage of a patient’s stay. No matter the patient’s age or state, the monitor can speak for them, even when they’re not able to speak for themselves.
AUSTRALIA
Revolution Surgical Pty Ltd
4/22 Rowood Road, Prospect NSW 2148
ABN: 63 165 643 434
NEW ZEALAND
Revolution Surgical New Zealand Ltd
378 Neilson Street, Penrose Auckland 1061 New Zealand
NZBN: 145-925-924
© 2018- Revolution Surgical

