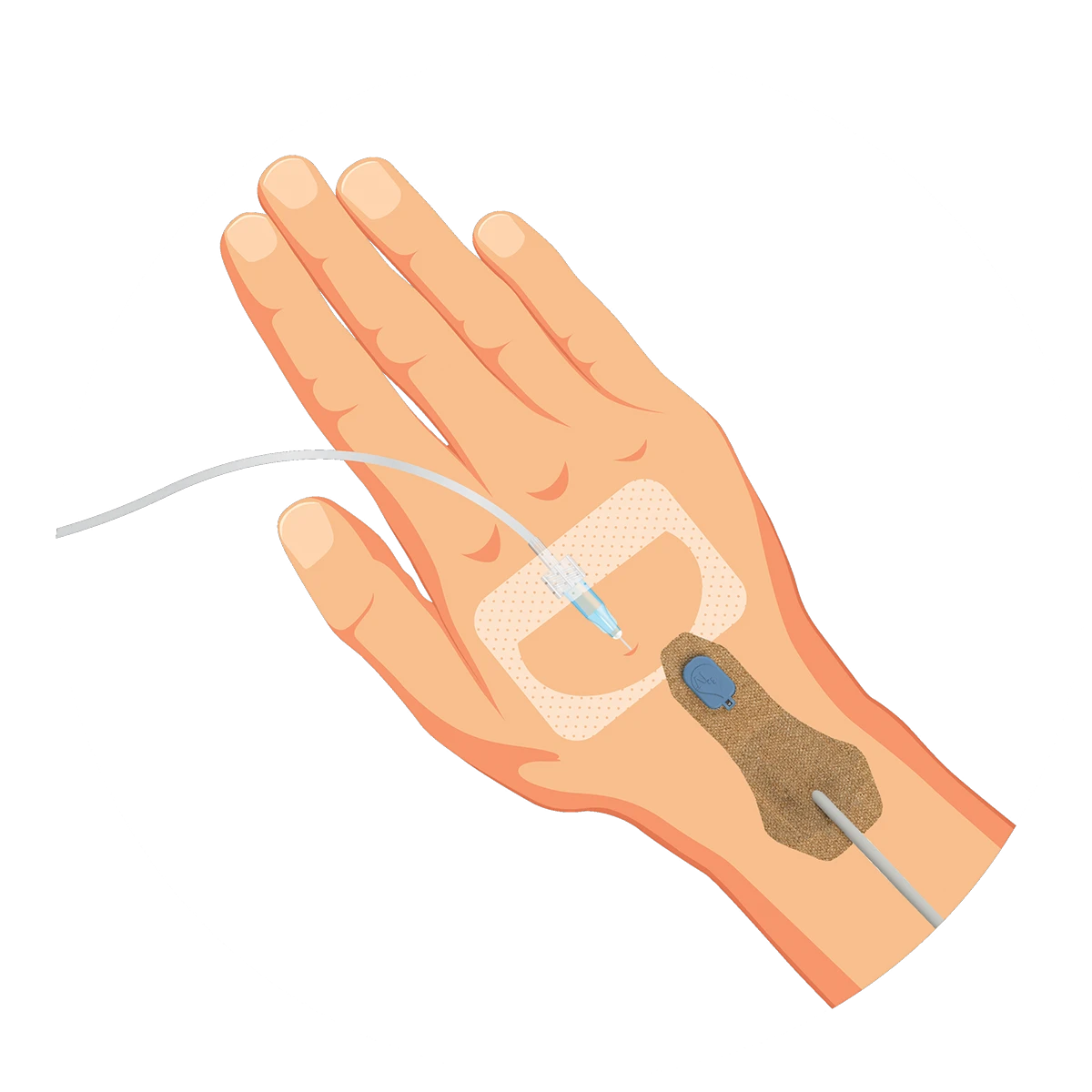
ivWatch was founded with one goal – to help clinicians provide the highest quality of care to every patient by detecting peripheral IV (PIV) infiltrations and extravasations at their earliest stage, when patient harm is highly preventable. Our non-invasive technology continuously monitors your patient’s IV status using advanced optics to notify you in real-time to the tissue changes that indicate a potential infiltration. ivWatch is here to help clinicians deliver optimal outcomes in vascular therapy.